Additional information
White Powder
143527-76-8
ACI-030615
222.1-222.8°C
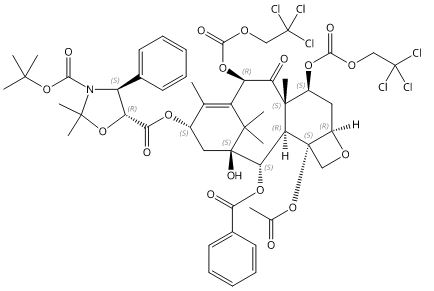
C52H59 Cl6NO18
1198.74
Ambient
2-8°C
Antineoplastic
Pharmaceutical Impurity Standards
7,11-Methano-1H-cyclodeca[3,4]benz[1,2-b]oxete, 3,5-oxazolidinedicarboxylic acid deriv
(4S,5R)-2,2-Dimethyl-4-phenyl-3,5-oxazolidinedicarboxylic Acid 5-[(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(Acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-4,6-bis[[(2,2,2-trichloroethoxy)carbonyl]oxy]-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl] 3-(1,1-dimethylethyl) Ester(Ditroc oxazolidine impurity)
13-{[(3-N-Boc)-2,2-dimethyl-4S-phenyl-1,3-oxazolidin-5R-yl]formyl}-10-deacetyl-7,10-bis{[(2,2,2-trichloroethyl)oxy]carbonyl} Baccatin III