Additional information
Free Base -86541-76-6
White Powder
86541-77-7
ACI-023402
107.7-109.0°C
C24H29N2O5Cl
460.95
Ambient
2-8°C
Antidiabetic
Pharmaceutical Impurity Standards
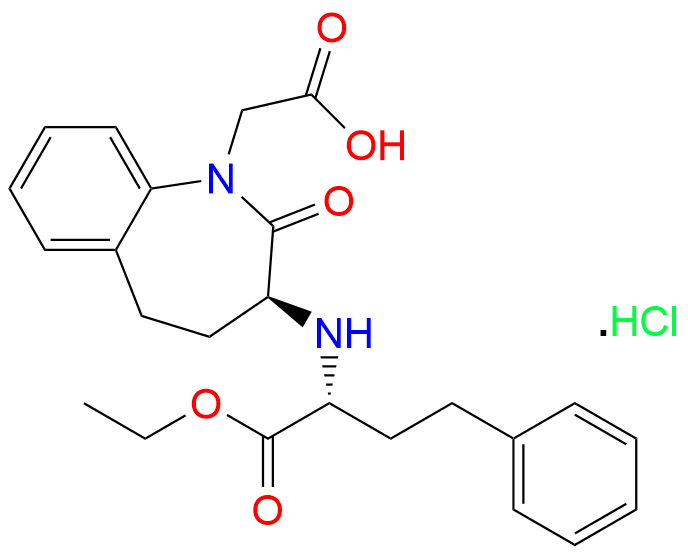
[(3RS)-3-[[(1SR)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid monohydrochloride
(3S)-3-[(1R)-1-(Ethoxycarbonyl)-3-phenyl propyl]amino]-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine-1-acetic acid monohydrochloride)
1H-1-Benzazepine-1-acetic acid, 3-[[(1R)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-2-oxo-, hydrochloride (1:1), (3S)
![3’,5’-O,O-DIPHOSPHORYL SOFOSBUVIR (10 mg) (Isopropyl [(S)-{[(2R,3R,4R,5R)-5-[2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl]-4-fluoro-2-({[{[1-isopropoxy-1-oxopropan-2-yl]amino}(phenoxy)phosphoryl]oxy}methyl)-4-methyltetrahydrofuran-3-yl]oxy}(phenoxy)phosphoryl]-L-alaninate)](https://analyticachemie.in/wp-content/uploads/uspimage-1.jpg)

